Introduction
Magnesium ribbon is a thin, silver strip of magnesium metal that burns with a bright white flame in air. It is often used in school chemistry experiments to show how chemicals react with each other, especially when they combine or give off heat.
Understanding how and why magnesium ribbon burns, why it is cleaned before burning, and what products form is very important for students and for exam questions based on practicals.
What is Magnesium Ribbon? (Definition and Properties)
Magnesium ribbon is a strip of metallic magnesium rolled into a thin, flexible form so that it can be easily cut and burnt in the laboratory. Magnesium itself is a reactive metal of Group 2 (alkaline earth metals) with atomic number 12 and symbol \(\mathrm{Mg}\).
- Colour of Magnesium Ribbon: When cleaned, the ribbon looks shiny and silvery white.
- Physical Properties: It is light in weight, soft enough to be bent, and conducts heat and electricity like other metals.
Because of its reactive nature and bright flame, magnesium ribbon is ideal for demonstration experiments in school labs and for ignition or flash effects in fireworks.
Colour and Appearance Before and After Burning
Before burning, magnesium ribbon is silvery white in colour if it has been properly rubbed with sandpaper and is free from the oxide layer. If it is not cleaned, a dull white coating is seen on its surface; this is magnesium oxide formed due to slow reaction with oxygen and moisture from air.
When magnesium ribbon burns in air, it produces:
- A flame that is very bright and white
- A white powdery ash of magnesium oxide \(\mathrm{MgO}\) that is basic in nature.
If moist red litmus paper is touched to the ash (after adding water), the litmus turns blue, confirming that magnesium oxide forms an alkaline solution (magnesium hydroxide) in water.
The Burning of Magnesium Ribbon in Air: Reaction & Observation
When magnesium ribbon is heated strongly in air or pure oxygen, it reacts with oxygen to form magnesium oxide.
Balanced Chemical Equation and Type of Reaction
The balanced chemical equation for the burning of magnesium ribbon in air is:
This reaction shows that two atoms of magnesium combine with one molecule of oxygen gas to form solid magnesium oxide.
- Type of Reaction: It is a combination reaction because magnesium and oxygen combine to form a single product, magnesium oxide.
- Exothermic or Endothermic: The reaction is exothermic; a large amount of heat and light energy is released, which is why the flame is so bright.
The key observation when burning magnesium ribbon in air is: “It burns with a bright white flame and leaves behind a white ash of magnesium oxide.”
Why Is Magnesium Ribbon Cleaned Before Burning? (The Oxide Layer)
In most exams often ask, “Why should a magnesium ribbon be cleaned before burning in air?” This point is directly related to how easily magnesium burns.
When magnesium ribbon is stored in air, a thin white layer of magnesium oxide forms on its surface. This oxide layer:
- Covers the fresh metal and acts like a protective coat.
- Prevents or slows down direct contact between magnesium metal and oxygen gas.
- Makes it difficult to ignite the ribbon quickly.
Why Sandpaper is Essential for Magnesium Ribbon Cleaning ?
Rubbing the ribbon with sandpaper to remove the oxide layer and impurities so that pure magnesium is exposed and the ribbon burns easily and quickly in air. The cleaned surface:
- Burns more easily and uniformly when brought near the flame.
- Increases the rate of reaction because the fresh metal is directly in contact with oxygen.
The Class 10 Practical: Burning Magnesium Ribbon Experiment
The “Burning of Magnesium Ribbon in Air” is a standard Class 10 Science practical. The aim is to study the burning of magnesium and identify the type of chemical change taking place.
Materials, Procedure, and Safety Points
Materials Needed: Magnesium ribbon, tongs, Bunsen burner/spirit lamp, watch glass, sandpaper, moist red/blue litmus paper.
Procedure (Simple Steps):
- Clean the magnesium ribbon with sandpaper until it becomes shiny and silvery.
- Hold one end of the ribbon with tongs and bring the other end into the flame.
- Observe: It ignites after heating and burns with a dazzling white flame.
- Collect the white powder (Magnesium Oxide) on a watch glass.
- Test the ash with moist red litmus paper after adding water (red litmus turns blue $\to$ product is basic).
Important Safety Tips:
- Never look directly at the bright flame produced by burning magnesium ribbon; it can strain the eyes.
- Always hold the ribbon with tongs, as it becomes extremely hot.
Summary of Observations and Inference
- During Burning: Produces brilliant white light and high heat.
- After Burning: A white, powdery solid (Magnesium Oxide) is left on the watch glass.
The inference is that the burning of magnesium ribbon is an exothermic combination reaction, as heat and light are produced, and a single new substance is formed.
Beyond Burning: Magnesium’s Reaction with Acids and Its Uses
Magnesium Ribbon Reaction with Dilute Hydrochloric Acid
Another popular experiment involves reacting magnesium ribbon with dilute hydrochloric acid ($\text{HCl}$):
This reaction shows that magnesium is more reactive than hydrogen and can displace hydrogen from dilute acids.
Uses Of Magnesium Ribbon And Magnesium Metal
Magnesium ribbon uses go beyond the school laboratory. In real life and industry, magnesium and its compounds have several applications.
- Demonstration and teaching: Burning of magnesium ribbon experiment is used in schools and colleges to teach combination reactions, exothermic reactions, and oxidation.
- Photography and fireworks: Earlier, magnesium ribbon and magnesium powder were used in flash photography and fireworks because of the intense white light produced on burning.
- Alloys and metals: Magnesium is used in light‑weight alloys for aircraft, automobiles, and sports equipment because it is strong yet lighter than many other metals.
- Chemical industry: Magnesium compounds like magnesium oxide and magnesium hydroxide are used in medicine (as antacids and laxatives), in construction materials, and as refractory linings.
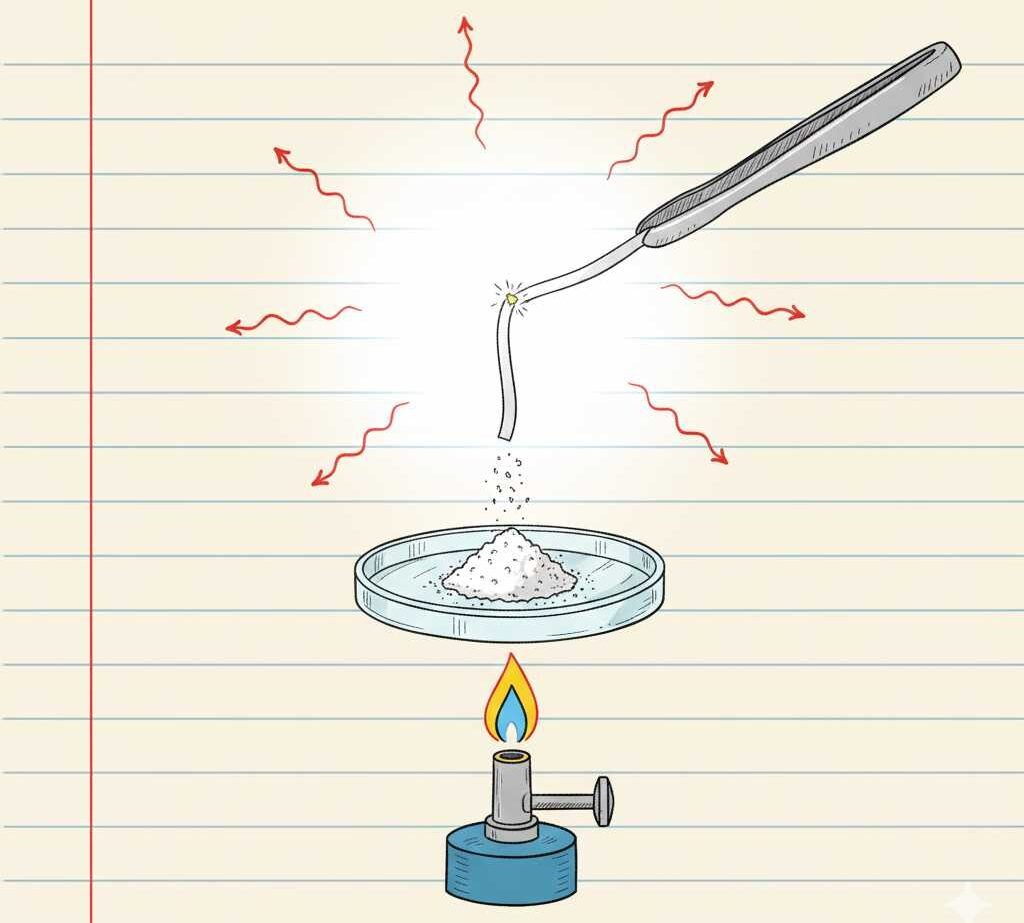
Diagram And Safety Points
A simple burning of magnesium ribbon diagram for notebooks shows:
- A Bunsen burner or spirit lamp at the bottom.
- A strip of magnesium ribbon held with tongs in the flame.
- Above the flame, bright light and arrows showing heat being released.
- A watch glass collecting the white ash of magnesium oxide.
While drawing, students should label “magnesium ribbon”, “burning in air”, “white light”, and “magnesium oxide ash” to make the diagram exam‑ready.
Important safety tips during the experiment:
- Never look directly at the bright flame produced by burning magnesium ribbon; it can strain the eyes.
- Always hold the ribbon with tongs, not with bare hands, because it becomes extremely hot.
- Perform the burning of magnesium ribbon in air experiment under teacher supervision and away from flammable materials.
- Do not touch the white ash immediately; let it cool before testing it with water and litmus.
Conclusion: Key Takeaways For Exams
To summarise the concept in exam language:
- Magnesium ribbon is a reactive, silvery metal strip that quickly forms a white oxide coating in air.
- Before burning, magnesium ribbon is rubbed with sandpaper to remove this oxide layer so that it burns easily in air.
- Burning of magnesium ribbon in air is a combination and exothermic reaction in which magnesium combines with oxygen to form magnesium oxide, a white basic oxide.
- The balanced chemical equation is 2Mg+O2→2MgO.
- Remember key keywords for answers: “cleaned before burning”, “removal of oxide layer”, “bright white flame”, “white ash of magnesium oxide”, and “exothermic reaction”.
Also read – How Propane Refrigerator Works?
Frequently Asked Questions (FAQs)
This FAQ section is designed to provide quick and clear answers to the most common inquiries we receive. We encourage you to click on a question to find the information you need. If you can’t find an answer here, please don’t hesitate to visit our Contact Us page for further assistance.
What is magnesium ribbon?
There is no guaranteed way to get periods overnight, but warm compresses, ginger tea, papaya, turmeric milk, and stress reduction may support menstrual flow. These remedies help relax the uterus and improve blood circulation, which might encourage periods if your body is already close to starting.
What is the colour of magnesium ribbon?
Silvery white and shiny.
Why should a magnesium ribbon be cleaned before burning in air or before any experiment?
To remove the magnesium oxide layer which prevents it from burning easily.
Why is magnesium ribbon rubbed with sandpaper or why do we rub magnesium ribbon with sandpaper before burning?
Rubbing with sandpaper removes the oxide layer and any dust or grease; it exposes fresh magnesium metal so that the burning of magnesium ribbon in air starts quickly and proceeds smoothly.
Write one observation when magnesium ribbon is burnt in air.
It burns with a brilliant white flame and forms a white ash of magnesium oxide.
Burning of magnesium ribbon is exothermic or endothermic?
Exothermic, because it releases a lot of heat and light energy.
What is the burning of magnesium ribbon equation?
$$\text{2Mg} + \text{O}_2 \to \text{2MgO}$$
Having any queries? – Do reach us at info@scivoyage.com