Introduction
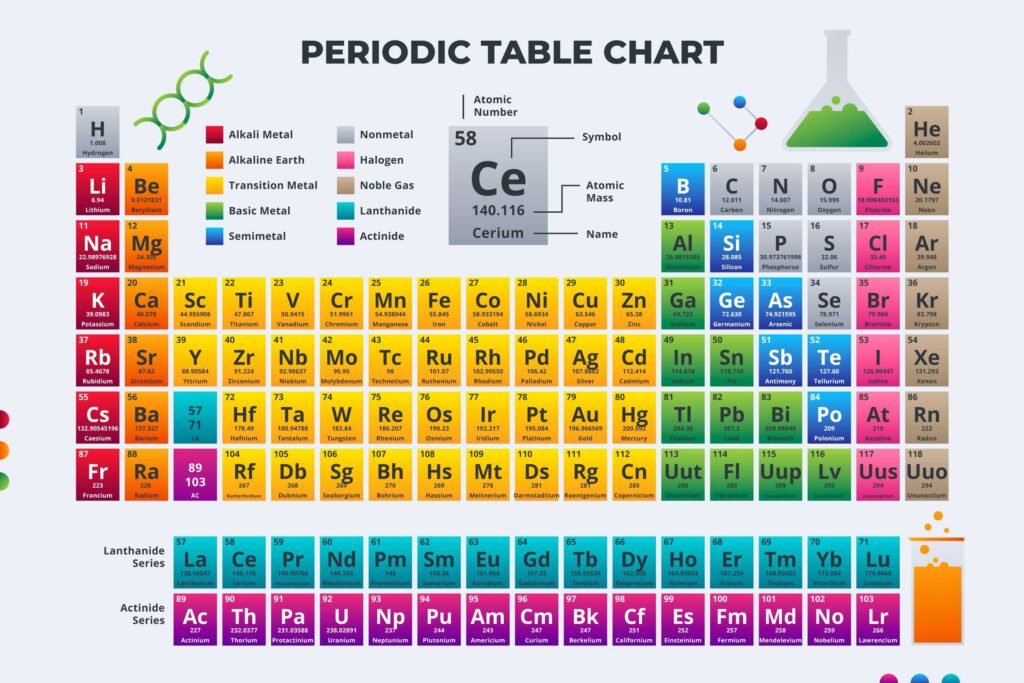
The Periodic Table a chart that organizes all known elements. It is recognized as the fundamental foundation of chemistry. For every science student, it is essential to be aware of and memorize the periodic table very well.
In the Periodic Table, each square shows the element’s:
- Name
- Symbol (Example: Fe for Iron)
- Atomic Number
- Atomic Mass
- Sometimes charges or electronegativity values
If you ever wondered how many elements are on the periodic table, the answer is 118 (as of today).
Mastering the names, symbols, and positions of the elements is crucial for understanding concepts like atomic structure, bonding, and chemical reactions.
While memorizing all 118 elements may seem challenging, especially for those preparing for competitive exams like CBSE, IIT JEE, IIT JAM, and NEET.
This guide explores the structure, mnemonics, and proven methods—the tools of a “Memory Rockstar”—to help you achieve effortless recall and the easiest possible way to learn the periodic table.
The Historical Evolution of the Periodic Table
Mendeleev’s contribution

Dmitri Mendeleev a Russian chemist, developed the initial table in 1869 after observing that specific elements shared similar characteristics. The table is often referred to as the Mendeleev Periodic Table.
It consists of the name, symbol, atomic number, and atomic weight of each element.
Understanding the Essential Structure
Before beginning any memorization technique, it is beneficial to comprehend the organization of the periodic table, as it is full of various repeating patterns that aid interpretation.
Systematic Arrangement of Elements
- Periods (Rows): The seven horizontal rows show the energy level of the electrons in an atom. The atomic number increases as you move from left to right.
- Groups/Families (Columns): The 18 vertical columns contain elements that are similar or have something in common, often sharing similar chemical behaviors.
Elements are generally categorized into Metals (residing on the left side, typically good conductors), Nonmetals (residing on the right side, typically poor conductors), and Metalloids (combining attributes of both).
Metals, Non-Metals, and Metalloids
- Metals are shiny and can bend easily. They also conduct electricity.
- Non-metals, on the other hand, don’t have these properties.
- Then there are metalloids, they’re kind of in-between, showing some traits of both metals and non-metals, but they don’t fully belong to either group.
Special groups: Transition metals, lanthanides, and actinides
Some metals are extra special. They’re called transition metals, along with the lanthanides and actinides. These metals are like the superheroes of the periodic table, really versatile and full of surprises. People use them a lot in fancy gadgets and for medical stuff.
Understanding the Core Periodic Table Trends
Once you know where an element sits on the periodic table, you can predict its behavior by understanding the major periodic table trends. These properties change predictably as you move across periods and down groups.
1. Electronegativity Periodic Table
Electronegativity is an element’s ability to attract electrons to itself in a chemical bond.
Trend: It generally increases as you move from left to right across a period and decreases as you move down a group.
The most electronegative element is Fluorine, found in the Halogens periodic table. The term periodic table electronegativity is a key search term for students.
2. Atomic Radius
This is half the distance between the nuclei of two atoms of the same element joined together.
Trend: It generally decreases from left to right across a period and increases down a group.
3. Ionization Energy
This is the energy required to remove an electron from a gaseous atom. It generally follows the opposite pattern of atomic radius.
Trend: It generally increases from left to right across a period and decreases down a group.
Charges and Valence Electrons in the Periodic Table
Understanding an element’s charge (or oxidation state) is the key to writing chemical formulas. This is directly related to its valence electrons periodic table—the electrons in the outermost shell.
Group 1 (Alkali Metals): 1 valence electron, typically forms a $+1$ periodic table charges.
Group 2 (Alkaline Earth Metals): 2 valence electrons, typically forms a $+2$ charge.
Group 17 (Halogens): 7 valence electrons, typically forms a $-1$ charge (they want to gain 1 electron).
Group 18 (Noble Gases): 8 valence electrons, stable with a charge of $0$.
Division of Blocks
In total, there are 118 confirmed elements. Approximately 90 elements are found in nature, while the others are man-made (artificial).
- s-block: On the far left, containing Group 1 (Alkali Metals) and Group 2 (Alkaline Earth Metals).
- p-block: On the right, encompassing Groups 13 through 18, which is an incredibly diverse area. Group 17 consists of Halogens, and Group 18 contains Noble Gases (excluding Helium).
- d-block: Located between the s-block and p-block (Groups 3 to 12). Groups 3 to 11 are also known as transitional metals.
- f-block: Known as Inner Transition Elements, divided into Lanthanides and Actinides.
Students do not need to memorize the synthetic elements, which typically include those with atomic numbers 104 to 118.
Mnemonic Mastery – Tricks for Every Block
Mnemonics, specifically acronyms and acrostics, are effective ‘first letter mnemonics’ that use associations, phrases, or sentences to aid recall. Knowing that Na periodic table is Sodium, Fe periodic table is Iron periodic table, and Ag periodic table is Silver periodic table is vital for understanding basic chemistry, and mnemonics make those quick recalls automatic. Similarly, Au periodic table is Gold.
A. Mastering the First 20 Elements
Memorizing the first 20 elements (Hydrogen to Calcium) is crucial for students. Mnemonics work because they link abstract data to familiar patterns.
Elements 1–10 (H to Ne):
Hi, He Likes Beer But Could Not Offer Full Nine Sodas.
Here, He Lies Beneath Bed Clothes, Nothing On, Feeling Nervous (H He Li Be B C N O F Ne).
Elements 11–20 (Na to Ca):
Naughty Maggie Always Sings Perfect Songs Clearly Around Kind Cats.
B. Mnemonics for s-Block Elements (Groups 1 and 2)
useful mnemonics for s-Block Elements for easy and quick learning in both English and Hinglish:
Group 1 (Alkali Metals: H, Li, Na, K, Rb, Cs, Fr)
| Category | Mnemonic Phrase | Elements Represented |
| English | How Little Nancy Knows Real Creative Facts | H, Li, Na, K, Rb, Cs, Fr |
| Hinglish | Hum Liye Na Kab Rab Cars Free | H, Li, Na, K, Rb, Cs, Fr |
Group 2 (Alkaline Earth Metals: Be, Mg, Ca, Sr, Ba, Ra)
| Category | Mnemonic Phrase | Elements Represented |
| English | Beautiful Mugs Can Serve Beer Rapidly | Be, Mg, Ca, Sr, Ba, Ra |
| Hinglish | Bhejiye Magar Candy Sari Baar Raat | Be, Mg, Ca, Sr, Ba, Ra |
Note: Group 1 in the source includes Ru; Group 2 includes Br. The mnemonics match these element lists.
C. Mnemonics for p-Block Elements (Groups 13–18)
Now mnemonics for quick recall of Periodic Table Groups 13–18 elements, organized neatly for study or classroom use:
Group 13 – Boron Group
Elements: B, Al, Ga, In, Tl
| Category | Mnemonic Phrase | Elements Represented |
| English | Bears Always Grab Insects Teals | B, Al, Ga, In, Tl |
| Hinglish | Baingan Alu Gaya India Thla | B, Al, Ga, In, Tl |
Group 14 – Carbon Group
Elements: C, Si, Ge, Sn, Pb
| Category | Mnemonic Phrase | Elements Represented |
| English | Cats Sing Generally Songs Publicly | C, Si, Ge, Sn, Pb |
| Hinglish | Chelo Simran Gae Sunny Pab | C, Si, Ge, Sn, Pb |
Group 15 – Nitrogen Group
Elements: N, P, As, Sb, Bi
| Category | Mnemonic Phrase | Elements Represented |
| English | No Potatoes As Substitutes Biscuits | N, P, As, Sb, Bi |
| Hinglish | Nai Park Assan Sub Bill | N, P, As, Sb, Bi |
Group 16 – Oxygen Group
Elements: O, S, Se, Te, Po
| Category | Mnemonic Phrase | Elements Represented |
| English | Old School Secrets Test Power | O, S, Se, Te, Po |
| Hinglish | Out Sun Say The Police | O, S, Se, Te, Po |
Group 17 – Halogens
Elements: F, Cl, Br, I, At
| Category | Mnemonic Phrase | Elements Represented |
| English | Fish Clean Brightly Inside Atlas | F, Cl, Br, I, At |
| Hinglish | Fir Chlo Break Interval Attack | F, Cl, Br, I, At |
Group 18 – Noble Gases
Elements: He, Ne, Ar, Kr, Xe, Rn
| Category | Mnemonic Phrase | Elements Represented |
| English | He Needs Art Keepers Xtra Runs | He, Ne, Ar, Kr, Xe, Rn |
| Hinglish | He New Area Kar Xeena Rani | He, Ne, Ar, Kr, Xe, Rn |
D. Mnemonics for d-Block (Transitional Metals)
A complete and easy-to-memorize list of Period 4 to Period 7 transition elements, with both English and Hinglish mnemonics, specially arranged for teaching or practice.
Period 4 Elements
Elements: Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn
| Category | Mnemonic Phrase | Elements Represented |
| English | Scott Ties Very Crude Mean Fedora Coats Nicely Cut Zincs | Sc to Zn |
| Hinglish | Sach Time Vicky Cross Man Fir Coffee Ni Chupa Zan | Sc to Zn |
Period 5 Elements
Elements: Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd
| Category | Mnemonic Phrase | Elements Represented |
| English | You Zipper Nobs Model Tocks Rub Rhinos Pads Again Code | Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd |
| Hinglish | Yeh Zara Nabhi Muh Toch Raha Rohit Pad Agar Code | Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd |
Period 6 Elements
Elements: Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg
| Category | Mnemonic Phrase | Elements Represented |
| English | Lucy Hafts That Weekly Report Osborne Iron Pats Authors Hugely | Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg |
| Hinglish | Lao Huffa Taaki Wo Rahe Ous Irsh Patli Aur Hoga | Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg |
Period 7 Elements
Elements: Ac, Rf, Db, Sg, Bh, Hs, Mt, Ds
| Category | Mnemonic Phrase | Elements Represented |
| English | Acid Rafts Dubiously Signed Bhai Has Meat Dish | Ac, Rf, Db, Sg, Bh, Hs, Mt, Ds |
| Hinglish | Aicha Rufus Dibba Sign Bhoby Hus Mat Das | Ac, Rf, Db, Sg, Bh, Hs, Mt, Ds |
E. Mnemonics for f-Block (Inner Transition Elements)
Here are Hinglish mnemonics to help memorize the Lanthanides and Actinides, divided into parts for clarity and ease:
Lanthanides Part 1
Elements: Ce, Pr, Nd, Pm, Sm
| Category | Mnemonic Phrase | Elements Represented |
| English | Certainly Pretty Neds Paiment Smartly | Ce, Pr, Nd, Pm, Sm |
| Hinglish | Chelo Priya Need Pamphlet Simple | Ce, Pr, Nd, Pm, Sm |
Lanthanides Part 2
Elements: Eu, Gd, Tb, Dy, Ho
| Category | Mnemonic Phrase | Elements Represented |
| English | European Goods Tables Die Horribly | Eu, Gd, Tb, Dy, Ho |
| Hinglish | Europe Gadha Tub Day Hotel | Eu, Gd, Tb, Dy, Ho |
Lanthanides Part 3
Elements: Er, Tm, Yb, Lu
| Category | Mnemonic Phrase | Elements Represented |
| English | Eric Teamed Yeb Loudly | Er, Tm, Yb, Lu |
| Hinglish | Error Time Yabba Lao | Er, Tm, Yb, Lu |
Actinides Part 1
Elements: Th, Pa, U, Np
| Category | Mnemonic Phrase | Elements Represented |
| English | They Pay Us Not Prolifically | Th, Pa, U, Np |
| Hinglish | Thoda Paani Us Napa | Th, Pa, U, Np |
Actinides Part 2
Elements: Pu, Am, Cm, Bk
| Category | Mnemonic Phrase | Elements Represented |
| English | Purple Amethysts Come Back | Pu, Am, Cm, Bk |
| Hinglish | Puri Aam Chamak Book | Pu, Am, Cm, Bk |
Actinides Part 3
Elements: Fm, Md, No, Lr
| Category | Mnemonic Phrase | Elements Represented |
| English | Farmers Midnight Now Large | Fm, Md, No, Lr |
| Hinglish | Famous Meds Na Lena | Fm, Md, No, Lr |
Some Tips to Learn the Periodic Table:
Also read – How Propane Refrigerator Works?
A. Break the Periodic Table into smaller sections
- Don’t try to learn all elements at once. Divide the table into small parts such as groups (families) or rows (periods).
- Learn 5-20 elements at a time, focusing on one group before moving to the next. This step-by-step approach makes memorization manageable.
B. Learn the elements in a song.
- Memorize the elements by singing songs made for the Periodic Table, or create your own tune. Mnemonics — funny or catchy phrases made from element symbols — are also great aids.
- For example, a phrase like “Happy Hector Likes Beer” helps remember element symbols in order.
C. Use color to learn element groups.
- Color-coding element groups like metals, non-metals, and metalloids helps your brain associate and remember them faster.
- Use colored pencils or markers to highlight groups when studying the table.
D. Using Acronyms and Short Forms
- When the Periodic Table becomes difficult to remember, try switching things up with different memorization techniques. Acronyms are a fun way to do this.
- Let’s take the metalloid elements as an example: Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), Polonium (Po). Here are two mnemonics to help remember them:
- Beyonce Sings Great And Acts Terribly Period.
- Boring Silly Germs Are Ants Telling Politics
E. Practice Makes Perfect
- Print blank periodic tables and practice filling in symbols and names. Start with one or two rows at a time and gradually add more.
- Repeated practice strengthens memory and builds confidence.
F. Testing Your Memory
- Test your memory by filling in a blank periodic table. After studying for a few days, try drawing the elements in their correct places from memory. Then compare it with a regular table to see how many you got right.
- Download periodic table apps onto your phone or tablet to help you study.
- There are many apps available that can help you learn about the elements, symbols, atomic numbers, and atomic weights. Some good ones to consider are:
- Memorize the Periodic Table,
- NOVA Elements,
- Periodic Table app by Socratica, and
- The Elements
- Oresome Elements.
- The Elements by Theodore Gray.
- Play online games to practice recalling the elements. Many websites offer games where you can match elements to their symbols or fill in missing information. These games can help improve your memory and score before a big test.
Conclusion
The periodic table isn’t simply a chart; it’s a way to see the cosmos. It may be simple and even entertaining to learn about if you do it the correct way. The first and most important step toward understanding is to learn the periodic table.
Students may effectively learn this important foundation by using visual tactics, mnemonic tricks, and regular, spaced repetition. This will make sure they know the “alphabet of chemistry.”
Also read – How Does a Microwave Work to Heat Food?
Frequently Asked Questions (FAQs)
This FAQ section is designed to provide quick and clear answers to the most common inquiries we receive. We encourage you to click on a question to find the information you need. If you can’t find an answer here, please don’t hesitate to visit our Contact Us page for further assistance.
How many elements are there in the periodic table?
The current periodic table has 118 elements, ranging from oganesson (atomic number 118) to hydrogen (atomic number 1).
What is the periodic table?
The periodic table is a chart that shows all the known chemical elements in order of their atomic numbers. It also shows how their properties reoccur.
As a technique to group “building blocks” of matter, you might think of it as putting them into rows (periods) and columns (groups) based on how similar they act.
For example, just as books in a library are categorized by genre and author, the elements in the table are grouped by how similar their chemicals are.
How to learn periodic table?
Here are some simple ways to study the periodic table:
Use mnemonic devices, which are phrases made out of the first letters of elements. For example, “Happy Henry Likes Beer But Can Not Obtain Food” for the first few elements.
Link each category (column) to its main property, such as metals or gasses.
Use flashcards, songs, or quizzes to practice.
Connect elements to things we use every day, such table salt for sodium and pencil lead for carbon.
For example, iron is needed to manufacture steel while hydrogen is found in water.
Who invented the periodic table?
Dmitri Mendeleev, a Russian chemist, came up with the periodic table in 1869 by grouping elements by their atomic weights.
How many groups are in the periodic table?
The current periodic table consists of 18 vertical columns known as groups.
How many periods are in the periodic table?
The current periodic table consists of seven horizontal rows known as periods.
How many metals are on the periodic table?
The periodic table contains around 89 metals, the majority of which are located to the left and center.
How many metalloids in periodic table?
The periodic table contains 6 to 7 metalloids (for example, boron, silicon, and arsenic), which are elements with characteristics of both metals and nonmetals.
Who discovered the periodic table?
Dmitri Mendeleev developed the basis for the contemporary periodic table, which organizes elements by atomic weight and predicts future ones.
How to remember periodic table?
- Use creative memory strategies such as songs, tales, and color-coded charts.
- Group elements into families (alkali metals, noble gases) and explain how they are used in everyday life.
- Practice regularly with online MCQ quizzes, games, and informative films.
Having any queries? – Do reach us at info@scivoyage.com











2 thoughts on “Easiest Possible Way to Learn Periodic Table”
Hi there, You’ve done an excellent job. I’ll definitely digg it and personally recommend to my friends. I’m confident they will be benefited from this site.
Great info and right to the point. I don’t know if this is really the best place to ask but do you people have any thoughts on where to employ some professional writers? Thank you 🙂